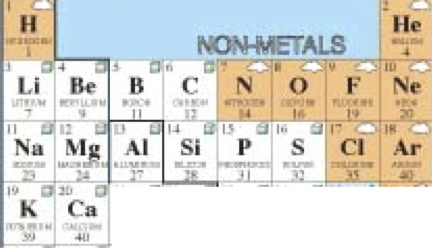
Week 2 Atomic Structure & Periodic Table
Task 1
In class mark
- Process Test
- Check Homework 5.1
- Complete Week 1 Task 3 - Analyse Periodic Trends of elements graphs
- Make observations of Rusting Experiment
Task 2 Introduction to the Atom
Go to Khan Academy http://www.khanacademy.org/science/chemistry/introduction-to-the-atom and watch the two videos on the page.
Remember to write notes and questions as explained in "Expectations"
- Elements and Atoms: How elements relate to atoms. The basics of how protons, electrons and neutrons make up an atom.
- Introduction to the atom: The atom, proton, neutron and electron
Remember to write notes and questions as explained in "Expectations"
Task 3 The Periodic Table
Go to Khan Academy http://www.khanacademy.org/science/chemistry/periodic-table-trends-bonding and watch the following videos on the page.
- Groups of the Periodic Table: Properties of alkali, alkaline earth and transition metals. Halogens and noble gases.
- Valence Electrons: Looking at valence electrons to figure out reactivity.
- Ionic, Covalent, and Metallic Bonds: Introduction to ionic, covalent, polar covalent and metallic bonds.
Task 4 Class exercise on electron arrangement in atoms of the first twenty elements of the periodic table
Fold your period table so that it looks like this;
Electron arrangement within atoms
http://freezeray.com/flashFiles/atomicStructure.htm
On your blank periodic table template
BORED yet?
Instead of drawing the arrangement of electrons within each atom;
http://freezeray.com/flashFiles/atomicStructure.htm
On your blank periodic table template
- Add atomic number in top left corner Add symbol in top right hand corner
- Show nucleus with number of protons
- Show electron energy shell with electrons
- First period may only contain one energy shell with a maximum of 2 electrons
- Second period may contain two energy shells with a maximum of 8 electrons
- Follow this pattern
BORED yet?
Instead of drawing the arrangement of electrons within each atom;
- Write the numbers of electrons in each shell. Put a comma to signify a different energy shell.
- Eg 2, 3
- 2, 8, 3
- Divide the job between the team members. Do this in the first twenty squares for elements with atomic number to 20.
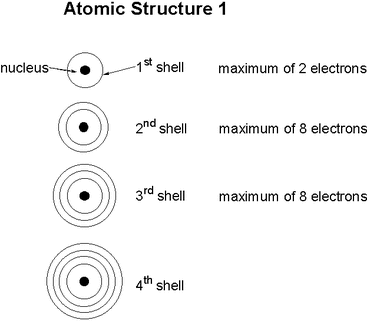
Analysing your Electron arrangement and electron configuration chart
- On your large Periodic table label the the Periods 1 to 4. Use the diagram to the left to explain what the period numbers mean.
- On your large Periodic table label the the Groups 1 to 8. Look at the outer electron shell and explain what the Group numbers mean.
The Element song
Task 5 Practical - Properties of Metals and Non-metals
Aim: To test and compare the appearance, malleability, hardness and electrical conductivity of some metals and non-metals.
Read through the group worksheet.
In your own book;
Leave your station neat and ordered for the next group.
Read through the group worksheet.
In your own book;
- Safety risks and their resolution
- Draw up a Results table; Observations of physical properties of metals and non-metals.
Leave your station neat and ordered for the next group.
- Summarise the properties of METALS and NON-METALS in terms of the physical properties of;
- appearance
- malleability
- hardness
- electrical conductivity